Throat cancer is becoming an epidemic, and sex could be why
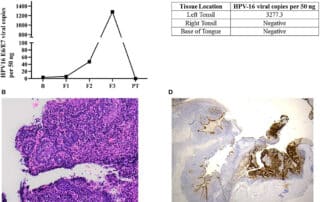
Source: www.sciencealert.com Author: Hisham Mehanna, Professor, Institute of Cancer and Genomic Sciences, University of Birmingham Over the past two decades, there has been a rapid increase in throat cancer in the west, to the extent that some have called it an epidemic. This has been due to a large rise in a specific type of throat cancer called oropharyngeal cancer (the area of the tonsils and back of the throat). The main cause of this cancer is the human papillomavirus (HPV), which are also the main cause of cancer of the cervix. Oropharyngeal cancer has now become more common than cervical cancer in the US and the UK. HPV is sexually transmitted. For oropharyngeal cancer, the main risk factor is the number of lifetime sexual partners, especially oral sex. Those with six or more lifetime oral-sex partners are 8.5 times more likely to develop oropharyngeal cancer than those who do not practice oral sex. Behavioral trends studies show that oral sex is very prevalent in some countries. In a study that my colleagues and I conducted in almost 1,000 people having tonsillectomy for non-cancer reasons in the UK, 80 percent of adults reported practicing oral sex at some point in their lives. Yet, mercifully, only a small number of those people develop oropharyngeal cancer. Why that is, is not clear. The prevailing theory is that most of us catch HPV infections and are able to clear them completely. However, a small number of people are not able to get rid [...]