Source: www.nextbigfuture.com
Author: Brian Wang
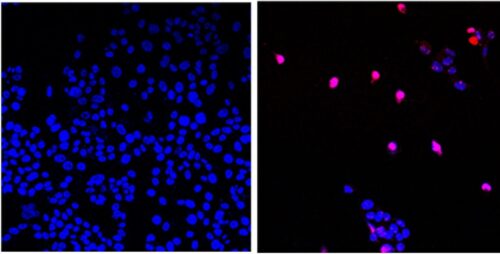
Above – The City of Hope-developed small molecule AOH1996 targets a cancerous variant of the protein PCNA. In its mutated form, PCNA is critical in DNA replication and repair of all expanding tumors. Here we see untreated cancer cells (left) and cancer cells treated with AOH1996 (right) undergoing programmed cell death (violet). (Photo credit: City of Hope)
Researchers at City of Hope, one of the largest cancer research and treatment organizations in the United States, today published a new study explaining how they took a protein once thought to be too challenging for targeted therapy, proliferating cell nuclear antigen (PCNA), and developed a targeted chemotherapy that appears to annihilate all solid tumors in preclinical research. As the scientists continue to investigate the foundational mechanisms that make this cancer-stopping pill work in animal models, they note that there is an ongoing Phase 1 clinical trial testing the City of Hope-developed therapeutic in humans.
Most targeted therapies focus on a single pathway, which enables wily cancer to mutate and eventually become resistant, said Linda Malkas, Ph.D., professor in City of Hope’s Department of Molecular Diagnostics and Experimental Therapeutics and the M.T. & B.A. Ahmadinia Professor in Molecular Oncology. However, the cancer-killing pill Malkas has been developing over the past two decades, AOH1996, targets a cancerous variant of PCNA, a protein that in its mutated form is critical in DNA replication and repair of all expanding tumors.
“PCNA is like a major airline terminal hub containing multiple plane gates. Data suggests PCNA is uniquely altered in cancer cells, and this fact allowed us to design a drug that targeted only the form of PCNA in cancer cells. Our cancer-killing pill is like a snowstorm that closes a key airline hub, shutting down all flights in and out only in planes carrying cancer cells,” said Malkas, senior author of the new study published in Cell Chemical Biology today. “Results have been promising. AOH1996 can suppress tumor growth as a monotherapy or combination treatment in cell and animal models without resulting in toxicity. The investigational chemotherapeutic is currently in a Phase 1 clinical trial in humans at City of Hope.”
AOH1996 has been effective in preclinical research treating cells derived from breast, prostate, brain, ovarian, cervical, skin and lung cancers and is exclusively licensed by City of Hope to RLL, LLC, a biotechnology company that Malkas co-founded and holds financial interest in.
The researchers tested AOH1996, a small molecule PCNA inhibitor, in more than 70 cancer cell lines and several normal control cells. They found that AOH1996 selectively kills cancer cells by disrupting the normal cell reproductive cycle. It targets something called transcription replication conflicts, which occur when mechanisms responsible for gene expression and genome duplication collide. The investigational therapy prevented cells with damaged DNA from dividing in G2/M phase and from making a copy of faulty DNA in S phase. As a result, AOH1996 caused cancer cell death (apoptosis), but it did not interrupt the reproductive cycle of healthy stem cells.
“No one has ever targeted PCNA as a therapeutic because it was viewed as ‘undruggable,’ but clearly City of Hope was able to develop an investigational medicine for a challenging protein target,” said Long Gu, Ph.D., lead author of the study and an associate research professor in the Department of Molecular Diagnostics and Experimental Therapeutics at Beckman Research Institute of City of Hope. “We discovered that PCNA is one of the potential causes of increased nucleic acid replication errors in cancer cells. Now that we know the problem area and can inhibit it, we will dig deeper to understand the process to develop more personalized, targeted cancer medicines.”
Interestingly, experiments showed that the investigational pill made cancer cells more susceptible to chemical agents that cause DNA or chromosome damage, such as the chemotherapy drug cisplatin, hinting that AOH1996 could become a useful tool in combination therapies as well as for the development of new chemotherapeutics.
As a next step, the researchers will look to better understand the mechanism of action to further improve the ongoing clinical trial in humans. Individuals interested in the Phase 1 clinical trial should review the eligibility requirements at clinicaltrials.gov. If eligible, call 626-218-1133 or visit City of Hope’s clinical trials webpage.
The Cell Chemical Biology study entitled “Small Molecule Targeting of Transcription-Replication Conflict for Selective Chemotherapy” was supported by the Department of Defense (W81XWH-11-1-0786, W81XWH-19-1-0326 under BC181474 and BC181474P1), National Institutes of Health/National Cancer Institute (R01 CA121289, R01 CA225843), St Baldrick’s Foundation, the Alex Lemonade Stand Foundation, Tobacco-Related Disease Research Program (TRDRP-T31IP626), Melanoma Research Foundation (MRF-717178), the ANNA Fund, RDL Foundation, Analytical Pharmacology Core supported by the National Cancer Institute of the National Institutes of Health (P30CA033572).

Leave A Comment
You must be logged in to post a comment.