Source: www.drugtargetreview.com
Author: Anna Begley (Drug Target Review)
Researchers at Icahn School of Medicine at Mount Sinai, US, have developed a new therapeutic approach by preventing the growth of metastatic tumours in mice to force cancer cells into a dormant state in which they are unable to proliferate. They hope that their findings will lead to new treatments that prevent the recurrence or spread of various cancer types, including breast cancer and head and neck squamous cell carcinoma (HNSCC).
In a previous study, scientists discovered that the ability of cancer cells to remain dormant is controlled by a protein called NR2F1. This receptor protein can enter the cell nucleus and turn numerous genes on or off to activate a programme that prevents the cancer cells from proliferating. NR2F1 levels are usually low in primary tumours but are elevated in dormant disseminated cancer cells. Levels of the NR2F1 protein then decline once more when cancer cells start proliferating again and form recurrent or metastatic tumours.
“We therefore thought that activating NR2F1 using a small molecule could be an attractive clinical strategy to induce cancer cell dormancy and prevent recurrence and metastasis,” explained Dr Julio Aguirre-Ghiso.
In the new study, detailed in the Journal of Experimental Medicine (JEM), the researchers used a computer-based screening approach to identify a drug, named C26, that activates NR2F1. The researchers found that treating patient-derived HNSCC cells with C26 boosted the levels of NR2F1 and arrested cell proliferation.
The researchers then tested whether C26 would prevent metastasis in mice. Animals injected with patient-derived HNSCC cells typically form large primary tumours that spread to the lungs after the original tumour is surgically removed. Treatment with C26 reduced the size of primary tumours, and, after surgery, further doses of C26 completely blocked the growth of metastatic tumours. Instead, the rodent’s lungs contained just a few dormant disseminated cancer cells unable to proliferate even after cessation of the treatment.
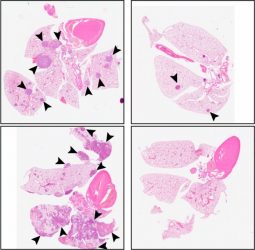
Compared with a control (left panels), C26 treatment (right panels) reduces the number of metastatic tumours in the lungs of mice injected with HSNCC cells. [credit: © 2021 Khalil et al.]
“Drugs that activate NR2F1 might be particularly useful in breast cancer,” proposed Dr Maria Soledad Sosa. “NR2F1 is highly enriched in oestrogen-receptor (ER)-positive tumours when compared to ER-negative tumours, and activating NR2F1 might be able to suppress reawakening of dormant cancer cells kept in that state by anti-oestrogen therapies.” However, because C26 treatment elevates the levels of NR2F1, the approach may also be useful for other cancers with inherently low levels of the receptor protein.
“Overall, our study reveals a mechanism-based and rationally designed strategy to exploit NR2F1-activated dormancy as a therapeutic option to prevent metastatic relapse,” Aguirre-Ghiso concluded.

Leave A Comment
You must be logged in to post a comment.