Source: Arch Otolaryngol Head Neck Surg. 2009;135(11):1133-1136
Author: Alexander Langerman, MD et al.
Complete Radiographic Response Correlates With Pathologic Complete Response in Locoregionally Advanced Head and Neck Cancer
Objective:
The role of neck dissection following chemoradiotherapy (CRT) for locoregionally advanced head and neck cancer is an area of active debate. Patients who have a complete radiographic response may not need dissection, and the extent of neck dissection necessary for those patients with residual disease is unclear.
Design:
Retrospective review of data from a prospectively collected database of patients with locoregionally advanced head and neck cancer treated as part of a phase 2 study of induction chemotherapy followed by concurrent CRT. The results of post-CRT neck computed tomography (CT) imaging and pathologic analysis of the neck dissection specimens were compared to evaluate correlation between radiographic and pathologic response.
Results:
Forty-nine patients underwent 61 hemineck dissections. Overall, 209 neck levels were dissected. Radiologic complete response in the neck was achieved in 39 patients, all of whom had pathologic specimens negative for tumor cells. Ten patients (20%) had a total of 14 neck levels with residual disease on CT imaging. Five (50%) of these 10 patients were found to have residual tumor cells on pathologic analysis. Tumor cells were contained only to those levels found positive on CT imaging; they were present in 7 (50%) of the 14 positive levels.
Conclusions:
Neck levels with residual disease on post-CRT CT imaging warrant removal. However, neck levels without evidence of disease on post-CRT CT imaging are unlikely to harbor cancer, which lends further support to the concept of basing neck dissection on post-CRT staging and performance of limited neck dissections for patients with limited residual disease.
Introduction:
The role of neck dissection following chemoradiotherapy (CRT) for locoregionally advanced head and neck cancer is an area of active debate. Traditionally, many centers recommended a planned neck dissection for all patients based on the extent of pretreatment neck adenopathy. This was supported by studies that demonstrate improved survival in patients who undergo posttreatment neck dissection.1-2 Proponents argue that post-CRT neck dissection may remove subclinical residual nodal disease within treated lymph nodes, even in the setting of a complete radiographic response. However, other studies demonstrated no survival advantage of neck dissection for those patients with complete radiographic response,3-4 and, in a pooled Radiation Therapy Oncology Group analysis, post-CRT neck dissection was associated with long-term toxicity.5
Throughout this debate, few studies have correlated radiographic and pathologic response to determine potential response to therapy. Therefore, we analyzed a population of patients with locoregionally advanced head and neck cancer uniformly treated with induction chemotherapy and concurrent CRT and post-CRT neck dissection, to correlate posttherapy radiographic findings to pathologic response.
Methods:
From November 1, 1998, to August 31, 2002, 222 patients with locoregionally advanced head and neck cancer (American Joint Committee on Cancer stage IV, except stage III base of tongue and hypopharynx cancers) were treated as part of a University of Chicago multi-institutional phase 2 study of induction chemotherapy followed by concurrent CRT. Patients with N2 or greater neck disease were recommended to undergo post-CRT neck dissection regardless of response to therapy. From the entire patient cohort, we restricted this analysis to patients treated at the University of Chicago (n = 130).
Treatment methods and outcomes for all patients have been previously reported.6 Briefly, all patients were treated with 2 cycles of induction carboplatin/paclitaxel followed by 5 cycles of concomitant hydroxyurea/fluorouracil/paclitaxel and 72 to 75 Gy of radiation. Patients underwent a post-CRT panendoscopy and biopsy of the primary site with frozen section analysis. Neck dissections were performed at the time of panendoscopy for patients with a complete response at the primary site.
Following CRT, all patients were restaged clinically and radiologically in a multidisciplinary conference with a contrast-enhanced computed tomography (CT) imaging of the head and neck. Patients with pretreatment N2 or greater neck disease or residual posttreatment disease underwent selective neck dissection. The extent of dissection was dictated by primary site and extent of prechemoradiation neck disease rather than postchemoradiation neck staging. At the time of the trial, a single, dedicated head and neck pathologist interpreted all neck dissection specimens.
Correlation of radiographic and pathologic response was performed in the following manner: post-CRT radiographic response for each patient was identified from study records. For completeness, CT imaging of patients with any evidence of lymphadenopathy was reviewed on a standard picture archiving and communication system workstation (Koninklijke Philips Electronics NV, Eindhoven, the Netherlands) that uses soft tissue windows. The location of pre-CRT and post-CRT lymphadenopathy was recorded for each patient. Lymphadenopathy was evaluated by means of criteria reviewed by Som.7 Nodes greater than 1 cm, with ill-defined or enhanced borders, or with areas of low attenuation suggestive of necrosis were considered pathologic. A complete response was defined as resolution of all evidence of radiographic disease. A partial response was defined as at least a 30% decrease in the sum of the longest diameter of the involved lymph node.
Detailed pathology reports of neck dissection specimens were reviewed for evidence of viable tumor cells. The location of the viable tumor cells was recorded for each patient and compared to the location of pre-CRT and post-CRT lymphadenopathy.
Data were entered onto an Excel spreadsheet (Microsoft Corporation, Redmond, Washington). The 2-tailed t test was used to determine statistical significance with a threshold of P.05. This study was conducted with the approval of the institutional review board of the parent trial.6
Results:
Of the 130 patients treated at the University of Chicago, 94 (72.3%) were initially staged with N2 or greater disease. Sixty-five of these 94 patients (69.1%) had complete response at the primary site. Of these 65 patients, 13 (20.0%) had neck dissections performed before referral to our institution for CRT and received no further neck treatment. The remaining 52 (80.0%) underwent planned neck dissection at a median of 10 weeks after CRT. Of these, 3 patients had incomplete neck level data on pathologic records and were excluded from further study. The remaining 49 patients underwent 61 hemi-neck dissections of 209 neck levels. The prechemoradiation neck stage was N2a in 2 (4.1%), N2b in 23 (46.9%), N2c in 10 (20.4%), and N3 in 14 (28.6%). No eligible patients refused neck dissection.
Radiographic Response to CRT
Radiologic complete response in the neck was achieved in 39 of 49 patients (79.6%), and 10 patients had radiographic residual disease. Radiographic residual neck disease was identified in 14 neck levels, most commonly in level II: 1 (7.0%) in level I, 9 (64.0%) in level II, 1 (7.0%) in level III, 1 (7.0%) in level IV, and 2 (14.0%) in level V.
Pathologic Response to CRT:
All patients with a radiographic complete response also had a pathologic complete response. Five (50.0%) of the 10 patients with radiographic residual disease were found to have residual tumor cells on pathologic analysis. Tumor cells were contained only to those levels found positive on CT imaging, being present in 7 (50.0%) of the 14 positive levels but none of the 26 negative levels in these patients. Tumor cells were found only in level I in 1 patient, only in level II in 3 patients, and in level II/III/IV in 1 patient (Figure). Pathologic positivity was not related to pre-CRT N stage (P = .24).
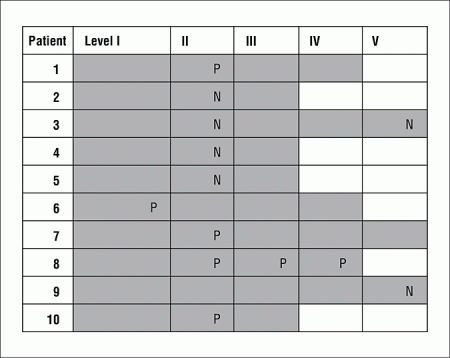
Figure. Distribution of suspicious-appearing lymph nodes on postchemoradiation computed tomography imaging and positive pathologic diagnosis in 10 patients who underwent neck dissection. Gray areas represent extent of neck dissection. P indicates pathologically positive; N pathologically negative.
Comment:
The necessity and extent of neck dissection following chemoradiation is controversial. At our institution and many others, the traditional method has been to perform a selective neck dissection appropriate for the primary site unless more extensive disease mandates more extensive dissection. For instance, an oral cavity tumor would warrant a supraomohyoid (levels I-III) neck dissection in the absence of disease beyond these levels. These criteria have been further refined by limitation of the neck dissection to only those patients with pre-CRT N2 or greater disease or residual disease post-CRT. In other words, in the majority of cases, the extent of dissection was dictated by primary site and extent of pre-CRT neck disease rather than post-CRT neck staging.
In the present study, all patients with a radiographic complete response also had a pathologic complete response. In those with a radiographic partial response, pathologically viable tumor cells were found in 50.0% of patients. Furthermore, pathologic residual disease was limited to levels restaged as positive on CT imaging.
Other series have reported a correlation of radiographic and pathologic complete response. Robbins et al8 analyzed 54 patients with advanced head and neck cancer who underwent neck dissection for evidence of persistent neck disease limited to a single level after combined intra-arterial chemotherapy and radiation. Their data demonstrated pathologically positive neck disease limited only to those neck levels that remained clinically positive by CT imaging in 52 of 54 patients (96.3%).
The above data, plus those included in this current report, suggest that radiographic residual neck disease should be dissected and that radiographically negative neck levels are unlikely to harbor disease. Although both the present data set and that of Robbins et al8 used CT imaging for restaging, considerable evidence has emerged in favor of positron-emission tomography (PET)9 or PET-CT.10-11 To the contrary, some have argued that PET adds little to CT.12 The relative merits of these modalities are beyond the scope of this article, but based on our and those of others, it appears that in centers where PET is unavailable or not used routinely, CT may be adequate to help surgeons decide whether or not to perform a post-CRT neck dissection.
Our data also suggest that a neck dissection limited to levels of radiographic involvement (ie, superselective neck dissection) constitutes adequate therapy following CRT. This concept was first suggested by Robbins et al.8 In their series, of the 2 patients whose CT scans failed to predict residual neck disease, 1 had neck disease in a contiguous level, which prompted the argument that it would have been discovered at the time of surgery, and which leaves only 1 patient for whom superselective neck dissection would have been inadequate. Robbins et al also reported that 100% of the radiographically positive neck disease was pathologically positive. Our data support this assertion because no patient was found to have disease other than at radiographically evident levels.
In a review article on management of the neck following radiation, Medina et al13 summarized what has remained otherwise unpublished data on 55 patients who underwent neck dissection for clinically or radiologically residual disease in the neck following radiation with or without chemotherapy. The full details with regard to neck level positivity were not reported, but all 5 patients with disease limited to level II on restaging had no pathologically positive disease beyond level II. Data were less convincing for patients with disease in multiple levels on posttherapy restaging, with 2 of those 10 patients having disease beyond that predicted by restaging. However, it is possible that not all of these patients were restaged radiographically. Overall, the yield of pathologically viable-appearing disease in the neck dissection specimens was 27%. Based on this data, Medina et al argued that disease limited to level II may be appropriate for superselective neck dissection.
The reliability of research into the response of the neck (and primary site) to CRT ultimately hinges on the pathologic interpretation in tissue from post-CRT surgical extirpation and two of us (A.L. and K.M.S.) are currently preparing a forthcoming formal review of the pathology of tumor response to CRT. We believe markers that indicate the progression of host response (eg, inflammatory markers and degree of fibrosis) as well as aspects of the tumor nidus (eg, nuclear changes and necrosis) can and should be incorporated into guidelines to standardize the interpretation of these specimens. Further careful study of radiographic partial responders without pathologic residual disease is warranted to identify characteristics that could limit surgery to those with higher risk of pathologic positivity.
This study is limited by its retrospective nature. Additionally, because the patients in this study all underwent neck dissections, the natural history of the neck disease cannot be determined, and it is unknown how the tumor detected in the neck pathologic specimens would have behaved in vivo. Furthermore, although a thorough pathologic analysis was conducted at the time of surgical extirpation, it is always possible that microscopic disease was undetected. Finally, it should be noted that, by protocol, only those patients who had a complete response at their primary site underwent the planned neck dissections analyzed in this study, and these results may not be applicable to patients who failed CRT at the primary site.
Conclusions:
The high percentage of tumor cells in radiographically positive neck specimens suggests that neck levels with residual disease on post-CRT CT imaging warrant removal. Furthermore, neck levels without evidence of disease on post-CRT CT are unlikely to harbor cancer, which lends further support to the concept of basing neck dissection on post-CRT neck staging and performing superselective neck dissections for patients with limited residual disease.
References:
1. Brizel DM, Prosnitz RG, Hunter S; et al. Necessity for adjuvant neck dissection in setting of concurrent chemoradiation for advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2004;58(5):1418-1423. FULL TEXT | ISI | PUBMED
2. McHam SA, Adelstein DJ, Rybicki LA; et al. Who merits a neck dissection after definitive chemoradiotherapy for N2-N3 squamous cell head and neck cancer? Head Neck. 2003;25(10):791-798. FULL TEXT | ISI | PUBMED
3. Liauw SL, Mancuso AA, Amdur RJ; et al. Postradiotherapy neck dissection for lymph node–positive head and neck cancer: the use of computed tomography to manage the neck. J Clin Oncol. 2006;24(9):1421-1427. FREE FULL TEXT
4. Clayman GL, Johnson CJ II, Morrison W, Ginsberg L, Lippman SM. The role of neck dissection after chemoradiotherapy for oropharyngeal cancer with advanced nodal disease. Arch Otolaryngol Head Neck Surg. 2001;127(2):135-139. FREE FULL TEXT
5. Machtay M, Moughan J, Trotti A; et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer. J Clin Oncol. 2008;26(21):3582-3589. FREE FULL TEXT
6. Salama JK, Stenson KM, Kistner EO; et al. Induction chemotherapy and concurrent chemoradiotherapy for locoregionally advanced head and neck cancer: a multi-institutional phase II trial investigating three radiotherapy dose levels. Ann Oncol. 2008;19(10):1787-1794. FREE FULL TEXT
7. Som PM. Detection of metastasis in cervical lymph nodes: CT and MR criteria and differential diagnosis. AJR Am J Roentgenol. 1992;158(5):961-969. FREE FULL TEXT
8. Robbins KT, Shannon K, Vieira F. Superselective neck dissection after chemoradiation: feasibility based on clinical and pathologic comparisons. Arch Otolaryngol Head Neck Surg. 2007;133(5):486-489. FREE FULL TEXT
9. Isles MG, McConkey C, Mehanna HM. A systematic review and meta-analysis of the role of positron-emission tomography in the follow up of head and neck squamous cell carcinoma following radiotherapy or chemoradiotherapy. Clin Otolaryngol. 2008;33(3):210-222. FULL TEXT | ISI | PUBMED
10. Rabalais AG, Walvekar R, Nuss D; et al. Positron emission tomography-computed tomography surveillance for the node-positive neck after chemoradiotherapy. Laryngoscope. 2009;119(6):1120-1124. FULL TEXT | ISI | PUBMED
11. Nayak JV, Walvekar RR, Andrade RS; et al. Deferring planned neck dissection following chemoradiation for stage IV head and neck cancer: the utility of PET-CT. Laryngoscope. 2007;117(12):2129-2134. FULL TEXT | ISI | PUBMED
12. Tan A, Adelstein DJ, Rybicki LA; et al. Ability of positron-emission tomography to detect residual neck node disease in patients with head and neck squamous cell carcinoma after definitive chemoradiotherapy. Arch Otolaryngol Head Neck Surg. 2007;133(5):435-440. FREE FULL TEXT
13. Medina JE, Vasan NR, Krempl GA. Management of the neck after treatment with radiation with or without chemotherapy. Curr Treat Options Oncol. 2007;8(3):261-264. FULL TEXT | PUBMED
Authors:
Alexander Langerman, MD; Colleen Plein, BA; Everett E. Vokes, MD; Joseph K. Salama, MD; Daniel J. Haraf, MD; Elizabeth A. Blair, MD; Kerstin M. Stenson, MD
Authors’ affiliations:
Author Affiliations: Sections of Otolaryngology–Head and Neck Surgery (Drs Langerman, Blair, and Stenson and Ms Plein) and Hematology–Oncology (Dr Vokes), Department of Radiation and Cellular Oncology (Drs Vokes, Salama, and Haraf), and Department of Surgery, Cancer Research Center (Drs Vokes, Salama, and Haraf), University of Chicago, Chicago, Illinois.
Author Contributions:
Dr Langerman had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Langerman, Vokes, Haraf, Blair, and Stenson. Acquisition of data: Langerman, Plein, and Haraf. Analysis and interpretation of data: Langerman, Plein, Salama, and Stenson. Drafting of the manuscript: Langerman. Critical revision of the manuscript for important intellectual content: Plein, Vokes, Salama, Haraf, Blair, and Stenson.

Leave A Comment
You must be logged in to post a comment.