Source: www.sciencenews.org
Author: Nathan Seppa
There are two vaccines that guard against human papillomavirus, and they are in rare company among medical inventions — the vaccines prevent cancer. Only the hepatitis B vaccine can make the same claim. Cancer-causing HPV can trigger abnormal cell growth on the cervix, and cervical cancer still kills up to 4,000 U.S. women each year. The virus is also implicated in cancers occurring in the anus and the throat. All told, according to a 2011 study, 29 percent of sexually active U.S. girls and women carry a potentially cancer-causing HPV infection.
Preteen and adolescent girls and boys are priority groups for vaccines that prevent human papillomavirus infection.
© Jessica Rinaldi/Reuters/Corbis
Back in 2006 and 2009, when the HPV vaccines Gardasil and Cervarix came onto the market, health officials dreamed of halting the spread of HPV, which is sexually transmitted, in a single generation. Scientists call such blanket coverage herd immunity — in which a pathogen gets vaccinated into oblivion, becoming so rare that even unvaccinated people are protected.
With such heady potential, Gardasil, developed by Merck, and Cervarix, created by GlaxoSmithKline, should be an easy sell. They rev up a potent immunity against HPV 16 and 18, the two types of the virus that account for most cases of cervical cancer. Gardasil also prevents most genital warts. The immunity the vaccines provide is many-fold better than the weak protection engendered by a run-in with the virus itself, and since approval, both vaccines have proven safe. A study of nearly 190,000 girls and women, published in 2012 in Archives of Pediatric and Adolescent Medicine, found that the shots’ most common side effects were mild skin infections and fainting.
But the hope for herd immunity against HPV anytime soon is fading fast in most of the West. By 2011, only 53 percent of U.S. teenage girls from 13 to 17, a target group for the vaccines, had received them.
“It’s a disaster,” says Andreas Kaufmann of Charité University Medicine Berlin, who sees the problem from the perspective of a biologist. “HPV is strictly species-specific. It only occurs in humans.”
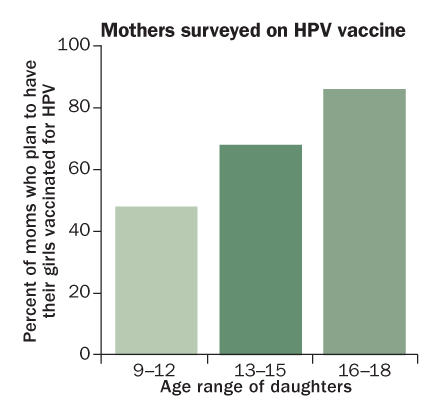
WHY WAIT?
Many U.S. mothers are reluctant to have preteen daughters vaccinated, even though that’s when protection is most likely to prevent a future HPV infection.
Source: J. Kahn et al/Pediatrics 2009
That means with mass vaccination, the virus would have no safe harbor in nature. “Theoretically, we could eradicate these HPV types, like we did smallpox,” he says. “We could end it.”
What’s the problem?
Most childhood immunizations are doled out in infancy. Although preteens and older kids routinely get shots or boosters for whooping cough, measles and meningitis, the HPV vaccines stand apart from those other shots like an unpopular kid.
For one thing, parents are uneasy about vaccinating a preteen against a virus associated with sexual activity. Researchers have found that some parents believe vaccination might lead to greater promiscuity. And a public scare about vaccines in general — including a false report linking the measles vaccine to autism — has contributed to the confusion. Not only that, but the vaccine is delivered in a three-shot regimen. Even among girls who get vaccinated, completing the course isn’t a certainty. Many U.S. preteen and teenage girls who start the course fail to get all three shots, and thus are less apt to be protected.
In the United States, responsibility for tracking kids’ HPV shots often falls to pediatricians, since the vaccine isn’t administered in schools. But pediatricians are notoriously overworked and — relative to many other physicians — underpaid. Doctors often need to cover vaccine costs up front to have them ready for patients, says Kevin Ault, a gynecologist at Emory University in Atlanta. Pediatricians also have to remind a patient to return for subsequent shots and often find themselves on the front line in contending with doubtful parents, says Noel Brewer, a health psychologist at the University of North Carolina in Chapel Hill. Instead of mass vaccinations in schools, the HPV vaccines depend on this hit-or-miss distribution system managed by individual doctors who, even if they advocate vaccination, may not want to cross parents. The result is often family indecision, procrastination and outright rejection.
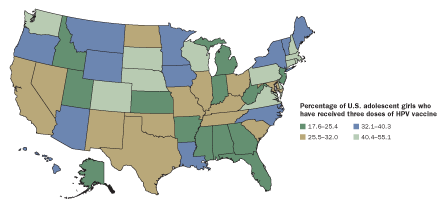
FALLING BEHIND
Health guidelines recommend the three-shot HPV vaccine for the best protection against cancer. But recipients don’t always complete the regimen. Compliance is worse in some states than in others.
Source: A. Jemal et al/JNCI 2013; Image: Geoatlas/Graphi-Ogre, adapted by E. Feliciano
Then there’s the behavior of the virus itself. The vaccines don’t work in people who have active HPV infections, and it’s difficult to know who those people are. The cancer-causing HPV types are stealthy, giving rise to phantom infections with no symptoms and an iffy risk of cancer far off in the future. These characteristics make the risks posed by HPV hard to grasp, says Christina Dorell, a physician at the Centers for Disease Control and Prevention. “With polio, people were getting sick and going to the hospital,” she says. “When the image of illness is removed from a group, you may have a little less sense of urgency coming from parents.”
Girls might see it differently, studies show. Doctors’ opinions matter to them. Those who receive a recommendation from a doctor are 2.6 times more likely to get vaccinated than girls getting no counsel, researchers reported in Pediatrics in 2011. Also, “there is no evidence of increased sexual-risk behavior, such as decreased condom use or earlier intercourse,” says Gregory Zimet, a clinical psychologist at the Indiana University School of Medicine in Indianapolis. Other work has found no increase in sexually transmitted diseases after HPV vaccination. “The whole [promiscuity] argument is false, actually,” Zimet says.
More likely, many parents are in denial about their teens’ sexuality, says Kaufmann: “Parents don’t believe that a 15-year-old daughter may already be sexually active.” But a 2010 U.S. survey found that at least 12 percent of 14- and 15-year-old girls had engaged in oral sex or intercourse or both.
One way to skirt the problem might be to vaccinate earlier. Health psychologist Jo Waller of University College London says focus groups show that parents like the idea of vaccinating girls as young as age 8 or 9, since that means skipping the chat about how the vaccine prevents sexual transmission of HPV. “They wouldn’t have to open that can of worms,” she says. Some countries do begin vaccinating at age 9, and several trials are under way testing the effectiveness of the shots at that age.
The fact of the matter is that the science underlying the HPV link to cancer is unassailable. German scientist Harald zur Hausen discovered the connection in the 1980s and was awarded a 2008 Nobel Prize for his efforts (SN: 10/25/08, p. 10). While Pap smears have averted most deaths from cervical cancer in the United States, the malignancy remains a leading cause of women’s cancer worldwide. Three shots of Gardasil or Cervarix protect against HPV types responsible for 70 percent of cervical cancers.
The other half of the equation
While cervical cancer is the most common malignancy prevented by the vaccines, in the United States nearly two-fifths of HPV-related cancers occur in men. That’s because HPV can cause cancers in the mouth or throat areas, and those strike both sexes. HPV is implicated in roughly 60 percent of oral cancers that affect the back of the tongue, throat and tonsils. Although many of these malignancies arise from alcohol and tobacco consumption, those types of cancers have declined in the United States in recent years even as overall oral cancer rates have stayed the same. HPV-related oral cancers account for the rise, particularly in men. In Denmark, the past decade has brought a shift in tonsil cancers, from 43 percent containing HPV to 75 percent.
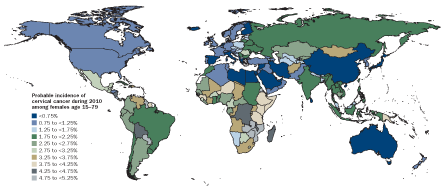
WORLD VIEW
HPV vaccines can prevent cervical cancers. Although roughly 40 countries worldwide now have HPV vaccination in their national health guidelines, few low-income countries — where cervical cancer remains a major problem — are in this group. However, pilot programs in some poorer nations indicate that the vaccine is well accepted, particularly when delivered at schools.
Source: M. Forouzanfar et al/Lancet 2011, adapted by E. Feliciano
Scientists established a link between oral cancer and HPV more than a decade ago when studies revealed HPV 16 lurking in many oral tumors. In 2007, researchers at Johns Hopkins University found that oral cancer patients were three times as likely as people without the cancer to have had six or more partners on whom they had performed oral sex. But there’s much still unknown about the dynamics of oral HPV transmission, says epidemiologist Marc Brisson of Laval University in Quebec. “Kissing may be involved.” He and others thinks that changing sexual practices may be behind the rise in oral cancers.
HPV vaccination is now recommended for boys in the United States (SN Online: 10/26/11). But because approval came later than it did for girls, only about 8 percent of boys ages 13 to 17, the initial target group, got at least one shot in 2011. As with girls, 11- to 12-year-old boys are the main vaccination target. But teenagers and young adults of both sexes can get the shots as part of a catch-up effort.
The HPV vaccines are given to prevent genital or anal HPV infections. Vaccine companies can’t make any claims regarding oral cancer because the vaccines haven’t been tested to prevent it. But the evidence is strongly suggestive.
“It’s time to start vaccinating boys,” says Margaret Stanley, a pathologist at the University of Cambridge in England. Boys and young men in Britain are not yet getting the shots, but Stanley and others are pushing for it. “It will protect 50 percent of the population, and not doing so would be truly discriminatory because that would include gay men, who are very much at risk of anal cancer,” she says. “And if you vaccinate boys, you start to get herd immunity.”
A shot at the herd
The slow launch of HPV shots in many countries is reminiscent of an earlier campaign that also could have stopped a sexually transmitted virus and the cancer it causes. “With the hepatitis B vaccine, we essentially lost a generation,” says Basil Donovan, a sexual health physician at the Kirby Institute and the University of New South Wales in Sydney. Slow implementation since the hepatitis B vaccine became available three decades ago has left the 350 million hepatitis B carriers worldwide at an increased risk of liver cancer.
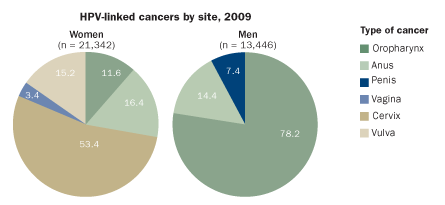
RISK ASSESSMENT
More than a dozen types of HPV can trigger abnormal tissue growth and malignancy in humans. The cancer burden affects women and men differently, as this chart of U.S. cases demonstrates.
Source: A. Jemal et al/JNCI 2013, adapted by E. Feliciano
Similarly, delayed HPV vaccination chalks up a daily cost as more teens become sexually active without protection. About 6 million new genital HPV infections occur each year in the United States, mostly in teens and young adults. Oral HPV infections go uncounted. Canada is faring better, but a study there found that while parents permitted their daughters to get hepatitis B shots in school at an 88 percent rate, only 65 percent consented to HPV vaccination. Germany has lagged behind some other European countries because shortly after the HPV vaccines were introduced, vaccine opponents raised questions about side effects of the shots. “Doctors stopped recommending it,” Kaufmann says.
Life is different in Australia. There, public health officials have now documented mass HPV vaccination and the first glimmers of herd immunity. Australian authorities have left little to chance, vaccinating preteen and teenage girls in schools since 2007. They mainly use Gardasil, which prevents genital warts, and such warts are vanishing in young women coming into city clinics. This year Australia began vaccinating boys, too, but herd immunity in them started showing up even before the first shot was fired into a boy’s arm. It seems that protecting girls means protecting boys.
Australia’s school-based program to vaccinate girls against HPV, mainly with Gardasil, is showing benefits for both sexes. Public health officials examining urban clinic records have documented a steady decline in genital warts. The findings hint at herd immunity.
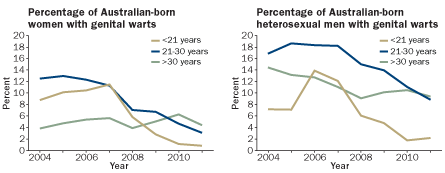
TRENDING DOWNWARD
Australia’s school-based program to vaccinate girls against HPV, mainly with Gardasil, is showing benefits for both sexes. Public health officials examining urban clinic records have documented a steady decline in genital warts. The findings hint at herd immunity.
Source: H. Ali et al/International Union Against STI World Congress in Melbourne 2012
Australia’s school-based vaccination program, which offers Gardasil free of charge for students, has set the pace for other nations. Between 2007 and 2009, 83 percent of preteen and teenage girls designated for vaccination had gotten at least one shot and 70 percent had received all three. More than half of young adult women got at least one shot, too.
Within two years of the program’s start, the rate of genital warts among girls and women was dropping every quarter at clinics monitored by scientists, Donovan says. Among women under age 21 examined at a eight clinics in Australia in 2011, less than 1 percent had genital warts, compared with more than 8 percent during the pre-vaccination years. Also in 2011, of 235 women who had been vaccinated against HPV, none had any warts, Donovan says. “Warts were a fairly obvious thing to monitor,” he says, since they can appear within months of infection. “In contrast, for cancer it’s measured in decades.”
Updated Australian data were released in late 2012 at conferences in Melbourne and San Juan, Puerto Rico. What really shocked attendees was the finding that genital warts in young men also dropped — from a range of 7 to 14 percent in pre-vaccination years to about 2 percent in 2011 — even though the widespread vaccination of boys hadn’t yet started in Australia.
The findings have changed how some people view HPV vaccination campaigns, Brewer says. “The data in Australia are just jaw-dropping.” Danish researchers recently reported substantial declines in warts as well.
Waller says the findings in heterosexual Australian men offer proof that there is herd immunity developing from having vaccinated women in Australia. “That leaves men who have sex with men as the main unprotected group,” she says.
The United States has special problems with school-based vaccination programs because there is no national health insurance that will cover the cost of the vaccine, as is the case in Britain, Canada and Australia. Still, a demonstration project in Denver is investigating a school-based program, says Lauri Markowitz, a medical epidemiologist at the CDC. While states can make vaccinations mandatory for school entry, mandates for HPV are rare, with only schools in Virginia and the District of Columbia requiring the shots.
In the long run, herd immunity remains the goal, and it’s not exotic. Anyone with children sees herd immunity in action. Routine childhood vaccines given to babies nowadays largely maintain herd immunity against scourges that beset previous generations. “The risk is near zero for an individual ever getting polio again,” Zimet says. “We continue to use the Salk vaccine to maintain herd immunity.”
The outlook for HPV may improve in coming years. Markowitz reported at the Puerto Rico meeting that among U.S. teenage girls, the rate of HPV infections of the types covered by the vaccines fell from 11.5 percent before vaccination introduction to 5.1 percent in the years after it, based on a nationwide database. And California public health authorities reported in 2012 that medical records show a substantial decline in genital wart diagnoses in girls in the post-vaccination years and a modest drop in boys.
Also, Merck is testing a new vaccine that covers the four HPV types in Gardasil as well as five others that can cause cancer. Math models suggest it could have a big impact on the HPV infection rate. “This seems like a great step forward,” says Zimet, who expects a nine-type vaccine to get cleared within a year or two.
Such a vaccine would help turn the tide, Stanley says. “You really want to prevent 90 percent of cervical cancers,” she says, “and that’s what it should do. Eventually, you wouldn’t need to screen for them [with a Pap smear]. You’d be looking for a rare disease. We ought to have no cervical cancer in 20 years.”
Other help might come financially. The Affordable Care Act — “Obamacare” — will eventually require insurance plans to cover all recommended vaccines, including HPV.
“The solution to the problem,” says Brewer, “is to improve the public health system we have. It may not rest solely on getting parents to act.” He suggests delivering HPV vaccines in schools and at pharmacies, like flu shots, and getting doctors to implement a system to recommend them routinely. “One or all of those would work,” he says.
Vaccinating against cancer
There are over a hundred types of human papillomavirus, says Robert Burk, a medical geneticist at the Albert Einstein College of Medicine in New York. But only about a dozen cause the vast majority of HPV-related cancers — and they take years or decades to do it. Still, those few viruses’ stealth makes them dangerous. Over millennia the viruses have perfected the art of colonizing humans and create very little stir when they do.
“In most of us the immune system recognizes the virus and deals with it,” says Margaret Stanley, a pathologist at the University of Cambridge in England. But these viruses can evade people’s immune reactions better than most. In some unlucky few, HPV triggers genetic mutations in the cells it infects, leading to abnormal cell growth and even to cancer. “A fraction of immune systems cannot handle these viruses well,” Stanley says. “We don’t know why.”
The Gardasil and Cervarix vaccines alert the immune system to the two most-studied cancer-causing HPV types, HPV 16 and 18. Together, these two viruses are thought to cause some 70 percent of cervical cancer. The vaccines against them appear effective, with evidence suggesting that even two doses may provide protection.
Research has now targeted several other cancer-causing members of the HPV family, and work is under way to test a nine-type vaccine that would add protection against HPV 31, 33, 45, 52 and 58. Gardasil and Cervarix may induce the immune system to develop partial cross-protection against these other HPV types. However, such cross-protection is not as strong as direct immunity.
Basil Donovan of the University of New South Wales in Sydney estimates that by the end of a young woman’s first sexual partnership, she has a 30 percent chance of having acquired an HPV infection. A 2011 study found that 43 percent of sexually active U.S. girls and women up to age 59 were carrying some type of HPV infection. Among U.S. men, the rate was about 50 percent for an HPV infection. In Germany and Denmark, the infection rate is roughly 35 to 40 percent among young women, says Andreas Kaufmann of Charité University Medicine Berlin.
“The vaccine has no effect on existing infections,” Burk cautions. But women who have been vaccinated before being diagnosed with an abnormal cell growth on the cervix — and treated to have the potentially precancerous growth removed — may benefit from that prior vaccination, researchers reported in BMJ in 2012. Vaccinated women were about half as likely as their unvaccinated counterparts to be diagnosed with a repeat lesion. Whether it’s useful to vaccinate a woman after she has cleared a lesion with surgery remains an open question, says gynecologist Kevin Ault of Emory University. But if it does help, those women would be prime candidates for vaccination since they would certainly be members of the unlucky few.
Timeline:
1940s
– George Papanicolaou develops Pap smear
1970s
– Harald zur Hausen’s team isolates HPV in genital warts
1980s
– zur Hausen’s team isolates HPV in cervical cancer
– Early vaccine development
1990s
– HPV vaccines developed
– HPV linked to oral cancers
– HPV found in 99.7 percent of cervical cancers
2000s
– Clinical trials of HPV vaccines
– Gardasil recommended for girls and young women (2006)
– zur Hausen wins Nobel Prize (2008)
– Cervarix recommended for girls and young women (2009)
2010s
– HPV vaccines recommended for boys and young men (2011)
Citations:
H. Bauer et al. evidence of human papillomavirus vaccine-effectiveness in reducing genital warts: An analysis of California public family planning administrative claims data, 2007-2010. American Journal of Public Health. In press, 2012. doi: 10.2105/AJPH.2011.300465
R. Bednarczyk et al. Sexual Activity-related outcomes after human papillomavirus vaccination of 11-to-12 year olds. Pediatrics. Volume 130, Number 5, November 2012, p. 1. doi/10.1542/peds.2012-1516.
J. Berkhof and J. Bogaards. Vaccination against human papillomavirus types 16 and 18: the impact on cervical cancer. Future Oncology. Volume 6, 2010, p. 1817.
B. Donovan et al. Quadrivalent human papillomavirus vaccination and trends in genital warts in Australia: analysis of national sentinel surveillance data. Lancet Infectious Diseases. Volume 11, 2011, p. 39. doi: 10.1016/S14733099(10)70225-5
C. Dorell et al. Human Papillomavirus Vaccination Series Initiation and Completion, 2008–2009. Pediatrics. Volume 128, Nov. 1, 2011, p. 830. doi: 10.1542/peds.2011-0950
C. Dorell et al. National and state vaccination coverage among adolescents aged 13-17 years – United Sates, 2011. Morbidity and Mortality Weekly Report. Volume 61, Aug. 31, 2012, p. 671.
G. D’Souza et al. Case–control study of human papillomavirus and oropharyngeal cancer. New England Journal of Medicine. Volume 356, May 10, 2007, p. 1944.
C. Fairley et al. Rapid decline in presentations of genital warts after the implementation of a national quadrivalent human papillomavirus vaccination programme for young women. Sexually Transmitted Infections. Volume 85, 2009, p. 499. doi: 10.1136/sti.2009.037788
A. Forster et al. Human papillomavirus vaccination and sexual behavior: Cross-sectional and longitudinal surveys conducted in England. Vaccine. Volume 30, July 13, 2012, p. 4939.
M. Forouzanfar et al. Breast and cervical cancer in 187 countries between 1980 and 2010: a systematic analysis. Lancet. Volume 378, October 22-28, 2011, p. 1461. doi.org/10.1016/S0140-6736(11)61351-2.
E. Garnaes. Oropharyngeal cancer and HPV in a large Danish cohort. 28th International Papillomavirus Conference – Puerto Rico, 2012.
M. Gillison et al. Prevalence of oral HPV infection in the United States, 2009-2010. Journal of the American Medical Association. Volume 307, Feb. 15, 2012, p. 693. doi:10.1001/jama.2012.101
S. Hariri et al. Prevalence of genital human papillomavirus among females in the United States, the National Health and Nutrition Examination Survey, 2003-2006. Journal of Infectious Diseases. Volume 204, Aug. 15, 2011, p. 566. doi: 10.1093/infdis/jir341
D. Herbenick et al. Sexual behaviors in the United States: Results from a national probability sample of men and women ages 14-94. Journal of Sexual Medicine. Volume 7, 2010, p. 255. doi: 10.1111/j.1743-6109.2010.02012.x
A. Jemal et al. Annual Report to the Nation on the Status of Cancer, 1975–2009, featuring the burden and trends in human papillomavirus (HPV)–associated cancers and HPV vaccination coverage levels. Journal of the National Cancer Institute. Volume 105, 2013, p. 175. doi: 10.1093/jnci/djs491. [Go to]
E. Joura et al. Effect of the human papillomavirus (HPV) quadrivalent vaccine in a subgroup of women with cervical and vulvar disease: retrospective pooled analysis of trial data. BMJ. Volume 344, online March 27, 2012, p. e1401. doi: 10.1136/bmj.e1401
J. Kahn et al. Mothers’ intention for their daughters and themselves to receive the human papillomavirus vaccine: A national study of nurses. Pediatrics. Volume 123, June 2009, p. 1439. doi: 10.1542/peds.2008-1536
N. Klein et al. Safety of quadrivalent human papillomavirus vaccine administered routinely to females. Archives of Pediatric and Adolescent Medicine. Volume 166, December 2012, p. 1140. doi:10.1001/archpediatrics.2012.1451
A. Kreimer et al. Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 Vaccine. Journal of the National Cancer Institute. Volume 103, Oct. 5, 2011, p. 1. doi: 10.1093/jnci/djr319. [Go to]
L. Markowitz. HPV vaccine impact on HPV prevalence in females in the United States: data from nationally representative surveys. 28th International Papillomavirus Conference – Puerto Rico, 2012.
S. Marur et al. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncology. Volume 11, August 2011, p. 781.
E. Simard et al. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA: A Cancer Journal for Clinicians. Volume 62, March/April 2012, p. 118. doi: 10.3322/caac.20141

Leave A Comment
You must be logged in to post a comment.