Source: http://www.sciencenews.org
Author: Susan Galdos
Rinse and spit.
Someday soon, doctors may join dentists in issuing these simple instructions. And before leaving the office, you might know whether you’re at risk for oral cancer. Additional tests on that same ptui may reveal whether you show signs of certain other cancers or diseases such as diabetes and Alzheimer’s.
Saliva — the frothy fluid that helps clean the mouth, digest food and fight tooth decay — carries many of the same proteins and other molecules found in blood and urine. Scientists have long been interested in mining a person’s mix of these compounds for clues to diagnosing diseases. Three years ago, these efforts got a boost when researchers completed a preliminary master list of the proteins found in spit — 1,166 of them.
Since then, scientists have nearly doubled the length of the protein list, while identifying changes in the salivary protein profile that are linked to disease. Other labs are delving into genetic material found in human saliva, looking for variations in gene activity that might signal disease risk.
Already, studies show that diseases such as breast cancer, type 2 diabetes and Alzheimer’s leave specific and identifiable signatures in saliva. Such biomarkers have also been found for Sjögren syndrome, an autoimmune condition that affects production of tears and saliva. And proteins known to be related to heart activity, including a handful whose levels are elevated during a heart attack, have also shown up in spittle.
Such findings — combined with the fact that saliva is quick, easy and painless to collect — may make spit the body fluid of choice to get an inside view of health, says David Wong, a dental expert at the University of California, Los Angeles who helped lead efforts to catalog spit’s proteins. “As a screening test, saliva is a latecomer,” Wong says. “But it’s catching up. The science is maturing and developing.”
Currently, most medical diagnoses are based on blood samples. That’s because serum, the clear part of blood, contains high amounts of proteins, genetic material and other molecules of interest. A bevy of studies have linked certain genes and proteins found in blood to specific diseases. But drawing blood requires trained clinicians and special equipment to handle and store the samples. Blood tests also mean frightening needles and long waits for test results.
Spit collection, on the other hand, is simple, if not glamorous. To get a sample, patients just drool into a vial or swipe a swab across their gums. Efforts are now under way to develop real-time detectors to diagnose disease from just a few drops of the slaver. Such test kits could be used in a dental office or emergency room and be carried onboard emergency response vehicles.
“The concept is that there are going to be various devices and capabilities that doctors, nurses and dentists — or patients themselves — can use to monitor health,” Wong says.
To make that happen, researchers are working to validate the biomarkers they’ve already discovered and to test tools that can be used on the fly.
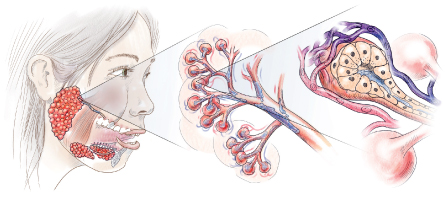
SALIVA'S SOURCEGlands on the bottom of the mouth and sides of the cheeks make saliva and empty it into the mouth via ducts. Within the glands, networks of capillaries take up proteins and other molecules from the blood, adding them to the gland-derived fluid. - Nicole Ranger Fuller
What’s in there
Saliva is mostly water, but its fluid content doesn’t come from the cola or the coffee that you drink. Instead, it comes from three major salivary glands located on the bottom of the mouth and sides of the cheeks, as well as smaller glands elsewhere in the mouth and throat. Within these glands, networks of capillaries surround the ducts that carry saliva to the mouth. As blood filters through the capillaries, specialized cells take up water, proteins and other molecules. This chemical brew gets mixed with the fluid produced by the glands, creating spit.
In 2004, Wong’s team at UCLA, along with groups at the University of California, San Francisco and the Scripps Research Institute in La Jolla, received funding from the National Institute of Dental and Craniofacial Research to find out what is in saliva. The list of ingredients includes more than 2,200 proteins, collectively called the “salivary proteome.”
About two-thirds of the proteins come from the salivary glands themselves. Some serve as protectors, Wong says, warding off microbes and other invaders that come into contact with the mouth. Other gland-produced proteins work to digest food, heal wounds or control the teeming hordes of bacteria residing in the mouth.
The remaining proteins come from other parts of the body, such as the liver, muscles or heart, and filter in through the blood. Though these proteins probably serve no specific role in the mouth, researchers can pick up on them to find out what’s going on in other parts of the body.
With that in mind, Timothy Griffin, a biochemist at the University of Minnesota’s Twin Cities campus, is looking to see how saliva proteins differ between healthy people and those with cancer. Studies have shown that saliva in patients with cancer may contain unexpected proteins. Other times, a protein may change in its abundance, showing up in fewer or greater numbers as the illness progresses, or it might get chemically modified, changing in shape or function.
Protein changes associated with a particular disease can serve as biomarkers for that disease, Griffin says. For the last three years, his group has been working to suss out protein biomarkers for oral cancer, collecting saliva samples not only from patients already diagnosed with the disease, but also from people who face an elevated risk, such as those with an oral lesion in a precancerous state.
Griffin says the idea is to identify subtle shifts in the molecular makeup of saliva that signal the transformation from a premalignant state to full-blown cancer.
Last year, his group reported in PLoS ONE that changes in the abundance of two proteins — myosin and actin — occur during this transformation. The scientists are now gathering more samples to see if the differences hold true for large numbers of people.
Such early markers are crucial in diseases such as oral cancer, where progression occurs in only a small percentage of cases, Griffin says. “Doctors could continue to watch a premalignant lesion to see if it’s at risk for transformation, and know when to intervene.”

A dollop of saliva on a chip like this one can be analyzed for disease-related proteins by a portable detector. Credit: Rice Univ.
Beyond the mouth
Griffin’s group is also looking at ways to better analyze saliva proteins that originate outside of the mouth to see what’s going on elsewhere in the body. The problem is, proteins that originate outside the mouth appear in saliva in quantities so small that searching for them becomes the equivalent of looking for a needle in a wet, stringy haystack.
In a study published in March in the Journal of Proteome Research, Griffin and colleagues used a technology called dynamic range compression, or DRC, to sort through the content of saliva samples collected from women who had breast cancer, looking for low-abundance proteins associated with the disease.
DRC employs various biomolecules to bind to the different proteins found in saliva. Proteins that bind remain in the sample, and those that don’t are washed away. Because proteins appearing in high numbers quickly saturate their binding baits, a large number are removed from the sample. Low-abundance proteins, though, bind fully to the bait and remain in the sample, boosting their proportion in the contents to be analyzed.
In the study, Griffin’s group collected saliva samples from 10 healthy women and 10 women with metastatic breast cancer. A portion of the samples from each group were left untreated and analyzed for their protein content; the rest were analyzed using DRC. The researchers identified twice as many different low-abundance proteins in the DRC–treated samples.
Among those proteins, Griffin’s group found a handful of biomarkers known from blood to be associated with metastatic breast cancer. “We knew that there were proteins in the blood that are diagnostic of these breast cancer patients,” he says. “But we wanted to see if we could see them in saliva.”
Griffin says the study serves as a proof of principle that saliva tests can detect proteins that change as a result of cancers in parts of the body besides the mouth. Such tests might someday be paired with other diagnostic measures, such as mammograms, to monitor women at high risk of breast cancer.
Saliva-based tests also hold promise as a screening tool for hard-to-diagnose diseases such as Alzheimer’s. Last year, scientists at the Neurodegenerative Diseases Biomedical Research Center in Madrid, Spain, found increased levels of a protein known to form the toxic brain plaques associated with Alzheimer’s in the saliva of Alzheimer’s patients. The protein may help diagnose the disease at early stages, when therapies are more effective, the team noted in BMC Neurology.
Wong’s lab is looking past proteins to ask what else in spit can be diagnostic. In recent years, the group has studied distinct bits of the genetic material messenger RNA in saliva. By focusing on this material, which carries the blueprints for protein synthesis, the scientists hope to determine when and where genes are turned on or off in various types of cells and tissues, and then relate this information to disease. So far, results show mRNA signatures for pancreatic cancer and breast cancer, as well as oral cancer and Sjögren syndrome.
Wong’s team is also looking at ways to diagnose disease by picking up on changes in the presence of microbial organisms that invade the mouth.
“Not every disease will reflect itself through the proteins, and not every disease will reflect itself through RNA,” Wong says. “If you want to look for biomarkers, the more targets you look at the better off you’ll be finding those targets that will be of interest.”
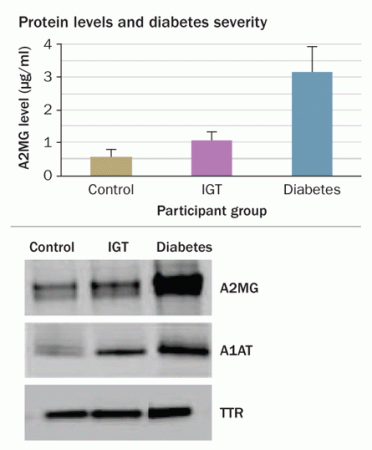
Levels of the protein A2MG differ among people without diabetes, with impaired glucose tolerance (IGT) and with diabetes (graph). Additional molecules (right) also show concentration increases with disease progression. Credit: P. Rao et al/Journal of Proteome Research 2009
Tools of the trade
With numerous signatures of disease now under study, scientists are working to develop instruments to detect and analyze the molecules found in saliva.
Wong’s group has designed a desktop device capable of simultaneously analyzing the protein and mRNA content of a sample within minutes. In a clinical trial, the device successfully detected both levels of interleukin-8, a protein associated with tumor growth and cell movement in numerous cancers, and levels of the mRNA that carries the blueprints for that protein. The study compared spit samples from 28 patients who had oral cancer with samples from 28 healthy people. Scientists could correctly identify patients with oral cancer based on elevated levels of these two biomarkers with 90 percent accuracy, findings published in 2009 in Clinical Cancer Research showed.
A dentist himself, Wong says that in the future dental offices might be equipped with such diagnostic devices, making dentists primary health-care providers. When patients come into a dentist’s office, in addition to getting a teeth cleaning or denture adjustment, they could be evaluated for diseases such as osteoporosis or cancer, he says. No needle pricks or embarrassing urine cups needed.
Other groups are developing devices that can be used on the go. Chemist John McDevitt of Rice University in Houston has developed a device, called a biotometer, that analyzes saliva samples, as well as other body fluids, on board emergency vehicles. Like programmers developing apps for an iPhone, McDevitt’s team has individual test cards customized for specific health conditions. Each card can look for multiple biomarkers associated with that condition.
The battery-operated analyzer weighs in at just under five kilograms, making it light enough to transport from place to place. Working like an ATM machine, the device reads information on the disposable test cards inserted into it. Each card contains a series of wells packed with tiny detection beads that act as microsponges to collect the biomarkers of interest.
McDevitt and his colleagues are already using the device to analyze spit samples to find out whether patients with chest pain are suffering a heart attack. A drop of saliva obtained from a gum swab goes onto the appropriate test card and is placed in the analyzer. If the saliva sample contains troponin T, a protein characteristic of a heart attack (it is released into the blood when heart cells die), the detection beads will emit a fluorescent color. The analyzer spots that color glow in the beads and indicates that the patient is indeed experiencing a heart attack.
McDevitt says he hopes saliva-based tests can detect heart attacks faster and more accurately than traditional methods. Of the millions of patients who visit emergency rooms with chest pain each year, only about half of those suffering a heart attack are immediately diagnosed using the electrocardiogram. The others must undergo additional testing, which can take anywhere from 90 minutes to several hours to process.
With the biotometer, clinicians could make a diagnosis within minutes instead of hours, McDevitt says.
The device is now being tested in a clinical trial in Houston, directed by Baylor College of Medicine cardiologists, to see how spit samples work in conjunction with the EKG to diagnose cardiac arrest patients. A second study is in progress on emergency vehicles in San Antonio.
Within spitting distance
Currently being manufactured by Force Diagnostics, a biotechnology firm in Chicago, McDevitt’s device will be made available for real-world applications within the next few months. The first units will be deployed for blood-based testing, targeting HIV infection in Africa. A 12-minute field test will replace a two-hour lab version, McDevitt says.
Initial applications for spit-based tests using the biotometer and other devices may appear within two to three years. Oral disease and gum disease tests will probably come online first. Widespread screening for conditions such as pancreatic cancer, breast cancer or heart attack may occur within five years.
These developments and other findings from various labs are laying the groundwork for a dramatic change in disease treatment, McDevitt says, allowing clinicians to move from reactive to preventive medicine.
As part of the heart attack trial, for example, his group is collecting information on protein changes associated with the risk of future heart attacks. Current diagnostic methods can’t easily detect the earliest stages of cardiac disease, he says, so the findings open up the possibility of using the biomarkers in saliva, or other body fluids, to detect health risks months or years before they become full-blown problems.
“I think we actually have a way to do it,” McDevitt says. “We’re seeing this in our trials right now.”
His optimism is supported by a study published in the Oct. 25 Journal of the American College of Cardiology. It showed that adjusting therapy to control levels of the protein NT-proBNP in the blood of heart patients could lower the rates of arrhythmias, stroke and heart attack. NT-proBNP is a known marker of cardiac distress, and is already detectable in spit. Because spit tests are cheaper and easier to administer than blood tests, clinicians might someday use them to monitor such patients.
But first, saliva-based biomarkers have to undergo vigorous tests to prove their reliability. That will require large-scale trials involving thousands of patients. Such trials are already evaluating markers associated with oral cancer, heart attack and Sjögren syndrome.
Obtaining scientific credibility is not the only hurdle to making saliva tests a clinical reality. If scientists can’t address some social stigmas, they may as well be spitting into the wind.
Spit doesn’t seem to have the respect of other body fluids, Wong says. “To say ‘I spit on your grave’ is considered a very negative statement.”
He hopes the saliva-based medical tests will help others see the value of this slippery fluid.

Leave A Comment
You must be logged in to post a comment.