Source: professional.cancerconsultants.com
Author: staff
A multicenter randomized trial has shown that patients with locoregionally advanced head and neck cancer receiving adjuvant Erbitux® (cetuximab) and radiotherapy who develop a rash have a better survival than patients receiving this therapy who don’t develop a rash. The details of this five-year follow-up of a Phase III randomized study were published early online in the Lancet Oncology on November 7, 2009.[1]
Standard treatment for head and neck cancer is largely determined by the stage and by the specific locations within the head or neck area where the cancer has spread. The patient’s overall medical condition is also a deciding factor. Treatment typically consists of radiation therapy, chemotherapy with surgery, or surgery alone.
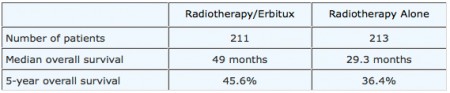
Erbitux is a monoclonal antibody that binds to the epithelial growth factory receptor (EGFR) and inhibits the receptor’s effects on cellular replication. Erbitux is currently FDA-approved for treatment of head and neck cancer. Researchers involved in an international study have previously reported that the addition of Erbitux to radiation therapy improves survival over radiation therapy alone in the treatment of head and neck cancer. The results of this randomized trial with a 54-month follow-up were published in the February 9, 2006, issue of the New England Journal of Medicine. This trial included 424 patients; approximately half were treated with Erbitux plus high-dose radiation therapy, and the other half received high-dose radiation therapy alone. This study now has a follow-up of more than five years.
The Following table summarizes some of the findings of this trial.
Table 1: Effect of the Addition of Erbitux to Radiotherapy in Head and Neck Cancer
Rash and reactions at the site of infusions were the most common side effects experienced by patients treated with Erbitux. Patients receiving Erbitux who experience a rash had 51% improvement in overall survival compared with patients receiving Erbitux without a rash.
The researchers concluded that the addition of cetuximab to radiation therapy is well tolerated and improves overall survival compared with radiation therapy alone in the treatment of patients with head and neck cancer.
Comments: This study with a longer follow-up demonstrates the lasting effect of adding Erbitux to radiotherapy for treatment of head and neck cancer. The association of rash with an increased response is of major interest.
Reference:
[1] Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncology [early online publication]. November 7, 2009.

Leave A Comment
You must be logged in to post a comment.