Source: Technology Review
Author: Emily Singer
Researchers call the experimental drug a major success for targeted cancer therapies.
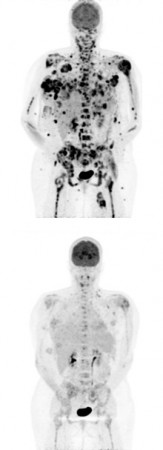
An experimental drug designed to block the effects of a genetic mutation often found in patients with malignant melanoma, a deadly cancer with few existing treatments, significantly shrank tumors in about 80 percent of those who carried the mutation. The findings, published Wednesday in the New England Journal of Medicine, signal a major success for so-called targeted cancer therapies, which are designed to block the effects of genetic mutations that drive the growth of cancer cells.
“This study is a major breakthrough in cancer treatment, and for metastatic melanoma,” saysMatthew Meyerson, an oncologist and researcher at the Dana Farber Cancer Institute in Boston. Meyerson was not involved in the study. “It’s a spectacular example of how genome-targeted therapies are beginning to help cancer patients.” The drug in the current study inhibits activity of a protein called BRAF, which is overactive in 50 to 60 percent of malignant melanomas.
Advances in genetic technologies over the last decade have allowed scientists to study the genetic mutations that underlie cancer in much greater detail. The result has been a new approach to drug design. Unlike chemotherapy, which can affect both healthy and cancerous cells and often triggers serious side effects, genetically targeted drugs act selectively on cancer cells that carry the mutation.
Only a handful of such drugs have been approved by the U.S. Food and Drug Administration, and most target rare mutations. The BRAF mutation, in comparison, is common. Discovered in 2002, the mutation disrupts regulation of the BRAF protein, making it continually active. The drug in the current study is under development by pharmaceutical giant Roche and Plexxikon, a startup based in Berkeley, CA. The drug is just one of a number of BRAF inhibitors currently in clinical tests.
Melanoma can be effectively treated with surgery in the early stages, but the prognosis is grim once the cancer has spread beyond the skin. The two currently available drugs work in only about 10 to 20 percent of patients. According to the new findings, 37 of 48 patients with the mutation responded to the new experimental drug, with their tumors shrinking by more than 30 percent. Tumors completely disappeared in three of those patients. About 30 percent of patients who took the drug the longest developed a specific type of squamous cell carcinoma, a tumor that usually doesn’t spread and typically resolves on its own.
Further studies are needed before the drug can be approved by the FDA. But because scientists can use genetic testing to select the patients for whom the drug is most likely to be effective, they require much smaller trials to show the drug works. Keith Flaherty, an oncologist at Massachusetts General Hospital who led the research, says the project reflects a new streamlined approach to clinical testing of cancer drugs that’s quicker and less expensive than traditional methods.
“The study highlights the potential power and importance of this type of approach,” saysAlexis Borisy, an entrepreneur in residence at Third Rock Ventures in Boston. “In a short period of time, we’ve gone from the discovery of the mutation to the design of a drug that is selective for that mutation, and which leads to dramatic clinical effects.” Borisy is the CEO of a startup, Foundation Medicine, that’s developing genetic screening tools to match cancer patients with the most appropriate drugs.
The new drug candidate does have limitations. Many patients develop resistance anywhere from three months to two years after beginning treatment, a problem that has occurred with other targeted cancer therapies as well. Scientists hope to overcome this problem by combining the drug with other compounds, an approach that worked with Gleevec, a targeted cancer drug used to treat some kinds of leukemia.
“We know some other genetic alterations that accompany BRAF, so the strategy would be to try to target more than one,” says Flaherty. “It’s like HIV, where doctors routinely use multiple drugs.” One such study, combining a BRAF inhibitor with a second drug that targets another protein in the same molecular pathway, has already begun. Researchers also aim to find the genetic variations that lead to resistance, which could ultimately help direct the most effective combinations.
The benefits of BRAF inhibitors are likely to extend beyond melanoma; the mutation is found in approximately 7 to 8 percent of all cancers, and tests of the drug in colorectal cancer are now underway. Scientists hope that studies such as these will lead to a new way to define cancer, relying on molecular characteristics rather than the tissue of origin.
A number of other clinical studies are underway for drugs targeting other mutations involved in various types of cancers. However, most of these mutations are much less frequent than BRAF. “A fundamental question that remains in the field is how many more targets like BRAF will there be,” says Flaherty. “That’s still a matter of discovery.”

Leave A Comment
You must be logged in to post a comment.